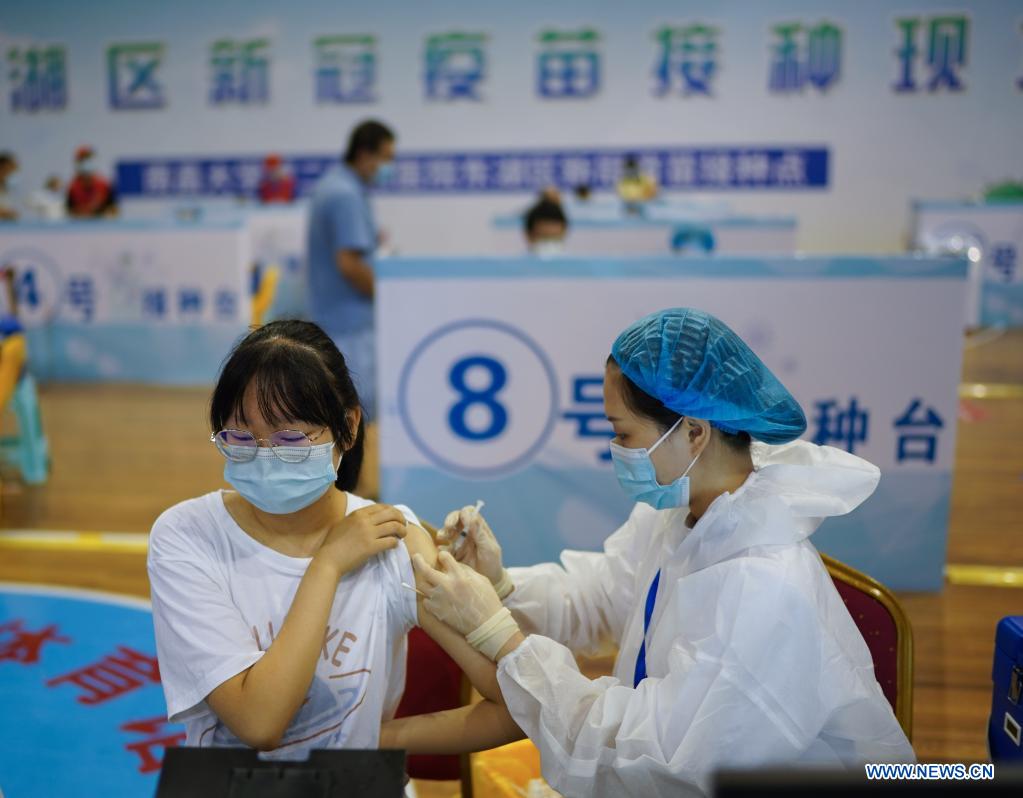
Scientists developing treatment, drugs for COVID-19

From discovering new antibodies to repurposing old drugs, scientists around the world are racing to find not only more effective vaccines but also medical treatment to fight the novel coronavirus and its more contagious variants.
Chinese scientists have reported that the 2B11 monoclonal antibody is highly potent in neutralizing the original strain and Alpha variant of SARS-CoV-2, according to a study published recently in the journal Cell Discovery.
In a follow-up experiment, the 2B11 antibody is also found to be effective against the Delta variant of the virus, said Yang Xiaoming, a researcher from China National Biotec Group-a subsidiary of Sinopharm-and the key scientist behind the study.
"This shows that 2B11 may have major application value in short-term prevention and early treatment of COVID-19 caused by the Delta variant," the release said, adding that the drug's application for clinical trials is currently underway.
A monoclonal antibody consists of lab-made proteins that mimic the immune system's ability to fight SARS-CoV-2 by targeting different parts of its spike protein, hence it can be used to directly neutralize the pathogen or prevent it from latching onto the ACE2 receptors and infecting human cells, according to the Journal of the American Medical Association.
As a result, scientists have hailed it as a promising treatment and prevention method against COVID-19. It also has the potential to work on a wide range of SARS-CoV-2 variants, which is important given the fact that many circulating mutated strains exhibit increased transmissibility and a capability to resist vaccine and infection-induced immunity.
However, this medication is typically given by an intravenous drip, so it must be administered in a facility with dedicated staff and resources, thus limiting its affordability and accessibility. It is also important to use this treatment during the early onset of the illness to ensure the best results, yet most COVID-19 patients, apart from the elderly and those with underlying health conditions, can recover on their own, thus raising the question of this treatment's usefulness.
Nevertheless, the journal states that a drug that can reliably prevent progression of COVID-19 would greatly reduce the concerns and uncertainty associated with SARS-CoV-2 infection.
"Establishing the therapeutic orprophylactic efficacy of monoclonal antibodies would be a major advance in the control of the COVID-19 pandemic," it said.
Last week, the United States Food and Drug Administration approved a preventive monoclonal antibody treatment for the estimated 3 percent of Americans who are immunocompromised. It was the first time an injectable coronavirus antibody treatment has been approved for the prevention of COVID-19 since the start of the outbreak.
Since the COVID-19 outbreak started, researchers have been having a hard time finding effective and accessible drugs to treat it due to the enormous amount of time and resources needed to discover and test new drugs.
As a result, some scientists hope to repurpose established drugs to find new therapeutic solutions with varying degrees of success.
As of July 31, the US FDA has approved only one medication, an antiviral drug called remdesivir, to treat COVID-19 in adults and children who are age 12 and older, according to the Mayo Clinic. The drug was originally designed to combat Ebola.
Anti-inflammatory drugs such as those used to treat rheumatoid arthritis, including baricitinib and tocilizumab, have either been approved or are authorized for emergency use to treat critically ill COVID-19 patients.
However, some existing drugs that were believed to be potent in the early days of the pandemic later proved to be ineffective, with some notable examples including the anti-malarial drugs hydroxychloroquine and chloroquine.
Vaccine development
On Thursday, Sinovac Biotech CEO Yin Weidong said during a forum that the company will apply in several countries for clinical research and emergency use for vaccines targeting the Gamma and Delta variants of SARS-CoV-2, the virus that causes COVID-19.
The new vaccines will be different from the two-dose CoronaVac inactivated vaccine produced by the company, said Yin. The company has also finished its clinical trial evaluating the potency of administering a third dose of CoronaVac as a booster shot, and the results are promising and would be published soon, he added.
Zhang Yuntao, vice-president of China National Biotech Group, also said that the company would submit applications for clinical research on vaccines against the Delta variant.
- Nation's space shuttle set to improve efficiency, cut costs
- China's new Preschool Education Law defines types of bans on individuals
- Heavy snowfall hits northern Xinjiang, fog and smog sweep north-central regions
- Eggs from Ningxia make fast, cool trip to Hong Kong
- Cultural tourism blooms in Greater Huangshan Region
- China's large neutrino observatory nears completion